AliveCor, which specializes in FDA-cleared personal electrocardiogram (ECG) technology, has announced findings from two new studies that further support the use of portable ECG devices to provide groundbreaking health monitoring capabilities.
A new Cleveland Clinic study — which marks the first time Apple Watch’s role in health care has been studied in a peer-reviewed manuscript — affirms KardiaBand’s ability to accurately detect atrial fibrillation (AFib), a leading cause of stroke. The Cleveland Clinic study, which has been accepted for publication in the Journal of the American College of Cardiology, set out to determine whether the KardiaBand for Apple Watch could differentiate between AFib and normal heart rhythm.
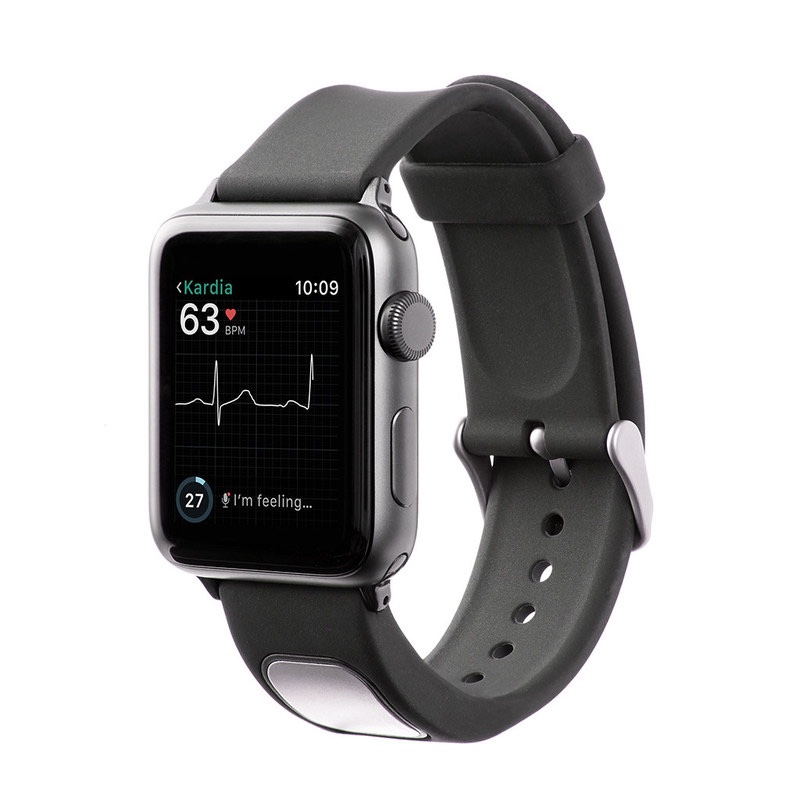
The $199 KardiaBand allows Apple Watch users to capture their EKG anytime, anywhere in order to quickly detect normal sinus heart rhythms and atrial fibrillation (AFib), the most common heart arrhythmia. The first FDA-cleared medical device accessory for Apple’s smartwatch, KardiaBand can record an EKG in 30 seconds with just a touch of its integrated sensor, according to AliveCor CEO Vic Gundotra. Results from the Kardia App are displayed on the face of Apple Watch.
AliveCor has also introduced SmartRhythm, a new feature within the Kardia app for the Apple Watch. It uses artificial intelligence in concert with inputs from Apple Watch’s heart rate and activity sensors to continuously evaluate the correlation between heart activity and physical activity. When SmartRhythm detects that heart rate and activity are out of sync, the device notifies users to capture an EKG with KardiaBand, or with KardiaMobile, AliveCor’s portable EKG reader.
Researchers found that KardiaBand successfully detected Atrial Fibrillation and normal sinus rhythm with an accuracy level comparable to physicians interpreting the same ECGs. In the study, the Kardia algorithm correctly interpreted AFib versus normal sinus rhythm with 93% sensitivity and 84% specificity. With physician review of KardiaBand recordings, sensitivity increased to 99%. Additional new research, presented today, reveals that, when paired with new artificial intelligence technology, AliveCor’s ECG device is able to perform non-invasive detection of high potassium levels in blood, a condition known as hyperkalemia.
The study used over two million ECGs linked with 4 million serum potassium values collected between 1994 to 2017, as well as prospective data from an AliveCor smartphone ECG device, to develop an AI algorithm to detect hyperkalemia. The sensitivity for hyperkalemia detection ranged between 90% to 94%.
Hyperkalemia is commonly associated with congestive heart failure, chronic kidney disease, diabetes, and with the medications used to treat these conditions. The condition, associated with significant mortality and arrhythmic risk, is often missed because it is frequently asymptomatic, making detection of hyperkalemia challenging. Until now, the only means of testing for hyperkalemia was through a blood test.
This capability, which over time may be commercialized through AliveCor’s KardiaBand for Apple Watch, could help to save more lives as AliveCor changes the way patients monitor their own healths, says AliveCor CEO Vic Gundotra. Both studies were presented at the American College of Cardiology’s 67th Annual Scientific Session.
