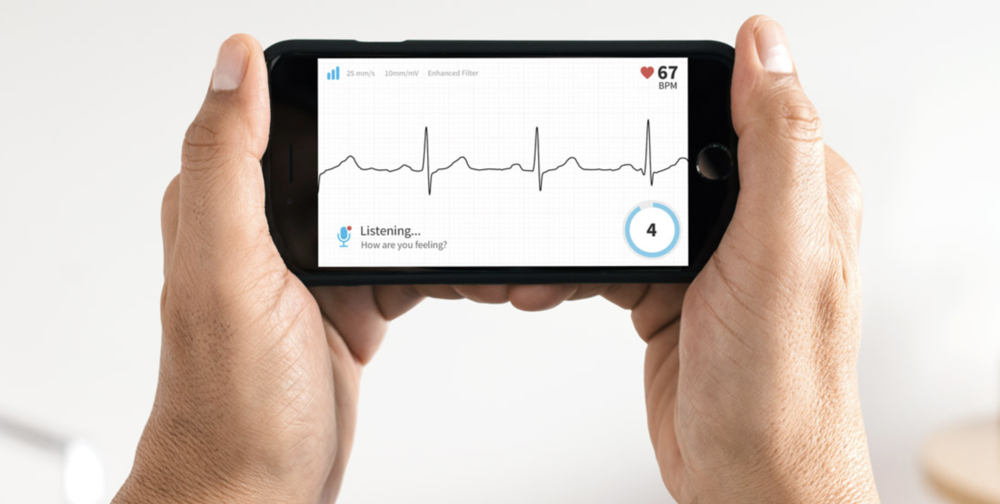
What a great time to be alive! Our smartphones have gone beyond just being communications devices and are rapidly morphing into “medical tricorders” that can provide insights into our health. For heart patients, getting an EKG meant one of two things — going to a doctor’s office and getting hooked up, or wearing a Holter monitor to capture heart activity data during normal life activities. AliveCor created the Kardia Mobile, a $99 FDA-approved device for taking instant medical-grade EKGs in 30 seconds using a small sensor pad and a smartphone or tablet.

In that 30-second reading, Kardia Mobile can determine if your heart is beating normally or if atrial fibrillation is detected. Heart activity data can be captured and saved, then shared with a doctor in seconds.
The accompanying iOS app provides a way to not only see the live EKG recording and pulse rate, but to record how you’re feeling at the time of the measurement. Once the measurement is complete, Kardia does an instant analysis showing possible findings. If readings are of concern, the result can be sent to Kardia’s Clinician Review service, or shared with your own cardiologist. A comprehensive review by a board certified cardiologist is just $19, while a quick review by a licensed cardiac technician is $9. The Kardia app now also captures blood pressure measurements, so it’s possible to see how readings vary over time and if hypertension is linked to abnormal EKG results.
Kardia Mobile has been approved for sale in the US, Canada, Australia, Germany, Ireland, India, Netherlands, New Zealand, Spain, Hong Kong, and the UK. The small, credit card-sized device can be used separately from the iPhone or attached to the back of an iPhone case.

If you think Kardia Mobile is cool, Apple Watch wearers will soon have the Kardia Band for Apple Watch. It’s a medical-grade mobile EKG watchband that works with a Watch app to capture an EKG on demand, providing instant detection of atrial fibrillation of normal heart rhythm. The device is currently pending FDA clearance.
